| HEAD OF TEAM | : | Dr. Eng. Jenny Rizkiana |
| TEAM MEMBERS | : | Prof. Dwiwahju Sasongko, Dicka Ar Rachim |
| OFFICIAL ADDRESS | : | Chemical Engineering Program, Labtek X, ITB Ganesha Campus |
| : | jr@che.itb.ac.id | |
| EXTENDED ABSTRAct | : |
Majority of Indonesian coals are low rank coal. Low rank coal gasification has low efficiency and low carbon conversion. However, gasification of low rank coal still can be accomplished by the addition of catalyst.
Co-gasification of coal with biomass can increase the carbon conversion, especially hydrogen [2]. Catalytic effect on co-gasification process caused by alkali and alkaline earth metal (AAEM) that can be found in biomass ash. In biomass, alkali and alkaline earth metal formed as carbonate. Alkali and alkaline earth metal that formed as carbonate can increase carbon conversion in gasification process through following reaction.
M2CO3 + 2 C → 2 M + 3 CO
2 M + 2 H2O → 2 MOH + H2
2 MOH + CO → M2CO3 + H2
where M is an alkali and alkaline earth metal
However, coal contains silicone in the form of silica, a compound that could deactivate catalyst. Risnes et al. [2003] found that silica could deactivate catalytic activity of AAEM by forming silicate, which has no catalytic activity. The present study is done to find the suitable condition to deash low rank coal. The conditions include acid solution type, acid concentration, temperature, and residence time. Deashing performance is evaluated by comparing the ash content of raw coal sample with the deashed ones.
The samples were leached with two kinds of strong acid solution, i.e. HF and HCl. The sample mass and solution volume used in all leaching experiments was 10 g and 250 ml, respectively. Experiments were performed at ambient temperature (25oC), medium temperature (47oC) and high temperature which were below the boiling points of the mixtures (70oC). Experiments using HCl were performed in a 500 ml glass beaker, while experiments using HF were performed in 500 ml Teflon beaker. All experiments were mounted on an electric hotplate with magnetic stirring device. There were three variations of reaction residence time of leaching, i.e. 2, 3 and 4 hours. After leaching, the mixtures were immediately filtered by Büchner funnel (vacuum filtration) for experiments using HCl, and it filtered by Polypropylene funnel for experiments using HF. The filtered cakes were washed with distilled water until the wash water became neutral. Filter cake residues were placed in an oven at 110oC overnight to remove water as a final drying step.
Leaching method using acid solution (HCl and HF) could decrease ash content in coal as shown in Fig. 1 and 2. The ash content of raw sample is 8.39%.
Ash content in coal sample could decrease approximately 20-23% weight compared to the ash content of raw sample by leaching method using HCl. Fig. 1(a) shows ash content of raw sample and leached samples at different HCl concentration. However, the increase of HCl concentration in leaching experiments did not affect the ash content significantly. Similar result was also obtained by Steel et al. [2001]. They found that dissolution of mineral matter isolated from coal with HCl slightly increased as the increase of HCl concentration. Fig. 1(b) shows ash content of raw sample and leached samples at different temperature. The increase of temperature in leaching experiments with HCl also did not affect the ash content significantly. Steel et al. also did leaching experiments with various temperatures, i.e. 20oC, 70oC, and 100oC. They found that ash content at temperature 20oC and 70oC not much different. However, the dissolution increases slightly when reaction temperature is raised to 100oC. Fig. 1(c) shows ash content of raw sample and leached samples at different residence time. Same with the previous result, the increase of residence time did not affect the ash content significantly.
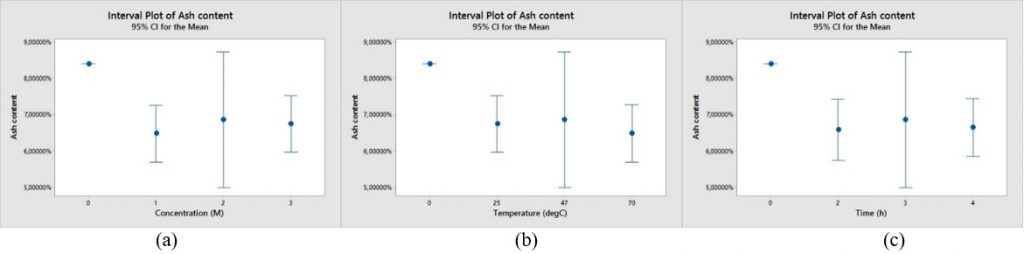
Fig. 1. Comparison of ash content range of leaching experiments using HCl with various (a) HCl concentration, (b) temperature, (c) residence time.
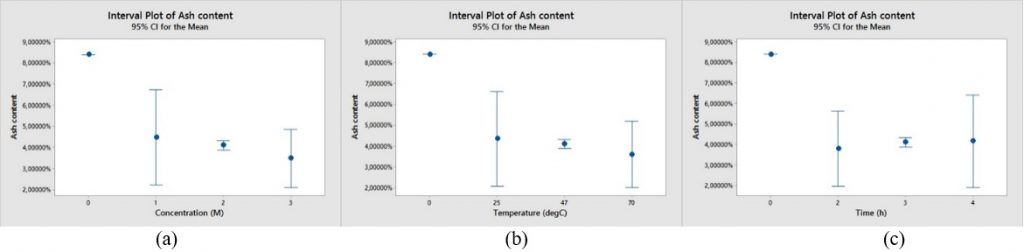
Fig. 2. Comparison of ash content range of leaching experiments using HF with various (a) HF concentration, (b) temperature, (c) residence time.
